Product CertificationsProduct CertificationsProduct Certifications
All products manufactured by Amicodenta are
All products manufactured by Amicodenta are  marked in conformity to Annex VII and Annex V of EC directive 93/42 and later amendments
marked in conformity to Annex VII and Annex V of EC directive 93/42 and later amendments
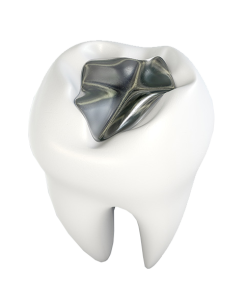
The Amicodenta amalgam capsule has good biocompatibility and operability. The filling material has good filling effects with low shrinkage and good sealing effect without looseness, falling off or breaking. The patients have no uncomfortable feeling during therapy.
Amico+ Amalgam capsuleAmico+ Amalgam capsuleAmico+ Amalgam capsule
HIGH STRENGTH AMALGAM
Amicodenta is primarily involved in the research and development, manufacturing and marketing of specialist dental materials. Amicodenta’s products are a combination of innovation and excellence to provide the ideal restorative materials for the dental profession